Diffusion mechanisms in matter
Diffusion is defined as the mechanism by which material is passed through the matter. Migration of atoms and molecules in gases, liquids and solids is constant over the period of time. In gases atomic movement is relatively rapid than liquid and solids.
Atomic movements are restricted due to bonding to equilibrium positions. However, Thermal vibrations occurring in solids do allow some atoms to move. Diffusion of metals and alloys in particularly important since most solid state reactions involve atomic movements.
Diffusion of unlike atoms in material also occurs. In this process, we can consider a Ni sheet bonded to a Cu sheet. At high temperature , Ni atoms gradually diffuse into the Cu atoms and Cu atoms migrate into the Ni.
Diffusion Mechanisms
There are two main mechanisms of diffusion of atoms in a crystalline lattice:
- Vacancy or substitutional diffusion.
- Interstitial mechanism.
Vacancy or substitutional Diffusion
In materials containing vacancies, atoms move or jump from one lattice position to another. This process is known as self diffusion. In self diffusion and diffusion involving substitutional atoms, an atom leaves its lattice to fill nearby vacancy. as diffusion continues , we have countercurrent flows of atoms and vacancies , called the vacancy diffusion.
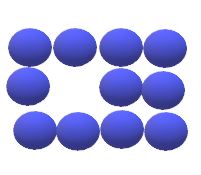
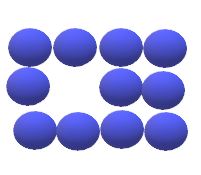
Vacancies in metals and alloys are equilibrium defects and , therefore ,some are present always to enable substitutional diffusion to atoms to take place. As the temperature of the metal increases , more vacancies are present and more thermal energy is available , and so the diffusion rate is higher at higher temperatures.
Interstitial diffusion
The interstitial diffusion of atoms ins crystals lattices takes place when atoms move from one interstitial site to neighbouring interstitial site without permanently displacing any of the atoms in the matrix crystal lattice.
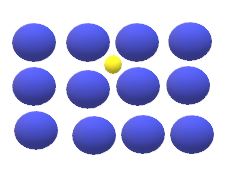
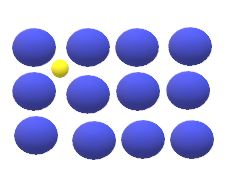
For the interstitial mechanism to be operative , the size of the diffusing atoms must be relatively small as compared to the matrix atoms , such ad , hydrogen, oxygen , nitrogen and carbon can diffuse interstitially in BCC and FCC.




