Basic vapour compression refrigeration cycle | Explained
A liquid boils and condenses at a temperature that depends on its heat, within the limits of its freezing point and critical temperature. The latent heat of evaporation must be obtained in boiling, and the latent heat must be given up again in condensing.
The basic refrigeration process uses a working fluid to boil and condense at different temperatures and thus at various pressures.
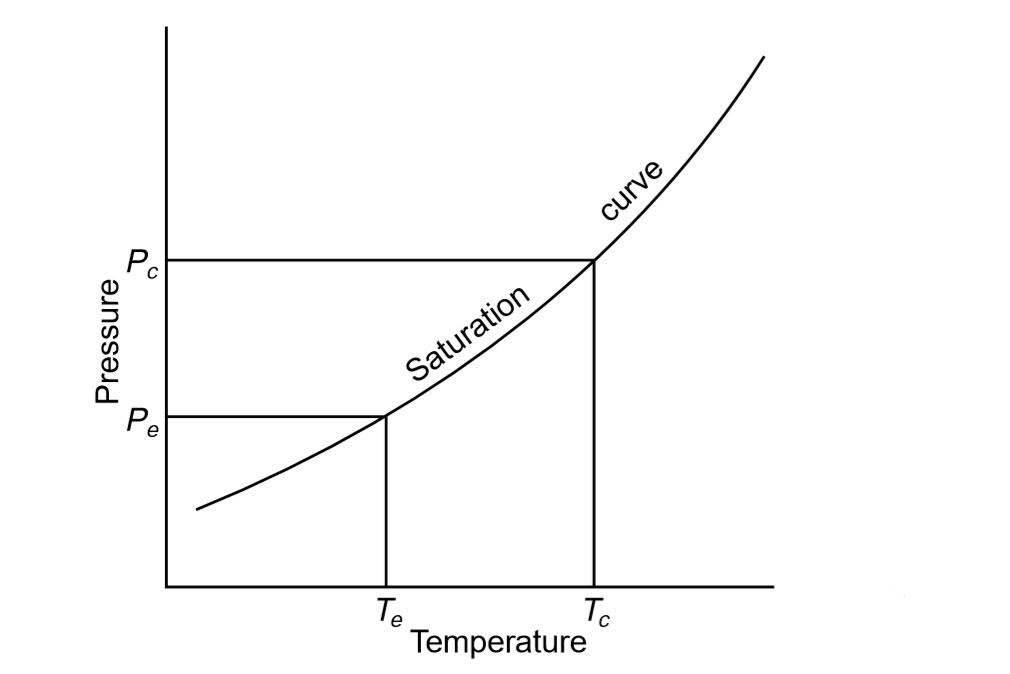
At lower temperature and pressure, heat is put into the fluid and so provides the latent heat for it to boil and transform to a vapor. This vapor is then compressed mechanically to a higher pressure and a corresponding temperature of saturation at which its latent heat can be discarded so that it changes back to a liquid.
The final cooling effect would be the heat transferred in the boiling or evaporating vessel to the working fluid, i.e. the difference in enthalpies between the fluid entering and the vapor exiting the evaporator. In a standard circuit, the pressures and enthalpies are shown below using the operating fluid Refrigerant 22, evaporating at – 5 ° C and condensing at 35 ° C.
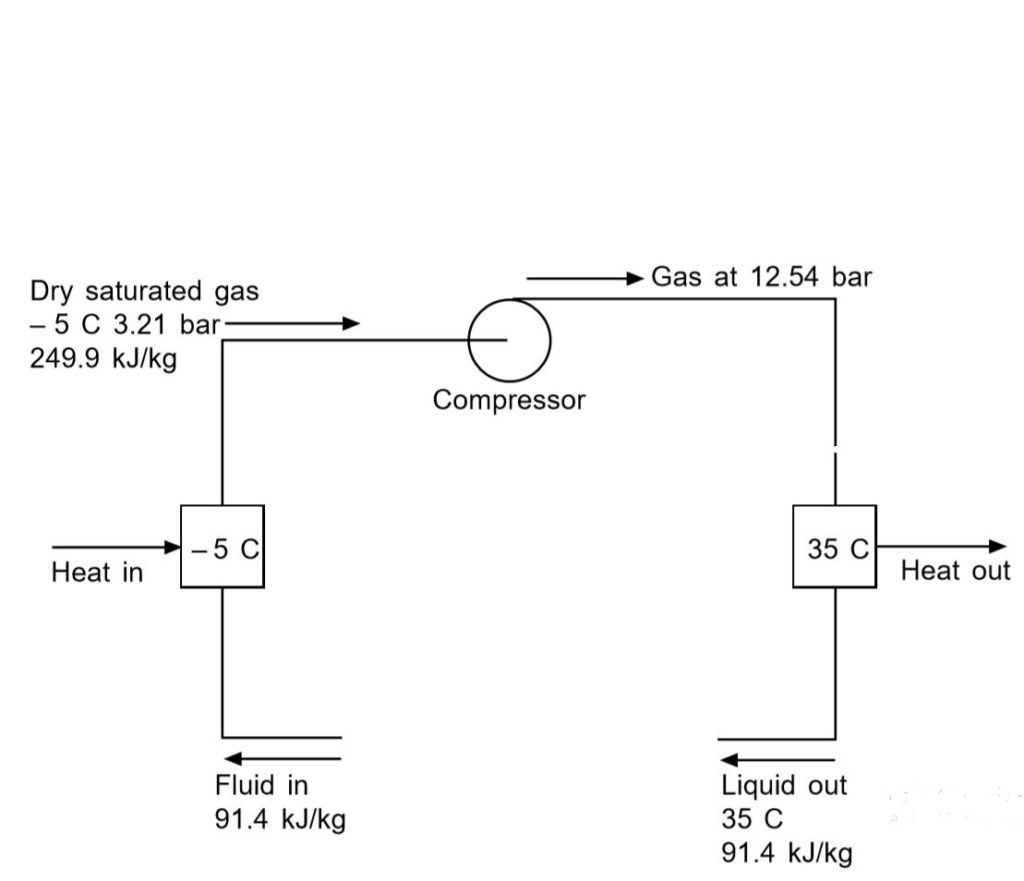
- Fluid entry evaporator enthalpy = 91,4 kJ / kg
- Saturated gas enthalpy leaving evaporator = 249,9 kJ / kg
- Cooling effect = 249,9 – 91,4 = 158,5 kJ / kg
To complete the circuit a working machine needs a connection between the condenser and the inlet to the evaporator. This contact would involve a pressure reducing and metering valve as they are at different pressures. Although the pressure reduction at this valve will induce a resulting drop in temperature, some of the fluid may flash off into vapor to release the energy for the cooling process. Therefore, the volume of the operating fluid rises by this quantity of flash gas at the valve, which gives rise to its name, the expansion valve.




Recent Comments