Defects in Solid Crystals
Crystals are the solids having highly organised structures of constituents like atoms, molecules etc. at microscopic level. They have crystal lattice spreaded in all directions.
All of the crystals have some defects and existence of fully pure crystal is not possible so defect in crystals is referred to the imperfections in the lattice arrangements or irregularity in lattice structure.
Defects are classified according to their atomic diameter or according to geometry and dimensions of the defects, so they are classified into three types:
- Point defects
- Line defects
- Surface defects
Point defects: Point defects are introduced due to movement of atom or ions in the crystal structure. This defect occurs when they gain energy by heating during processing of the materials by addition of impurities or by doping . Point defects are of 4 types:
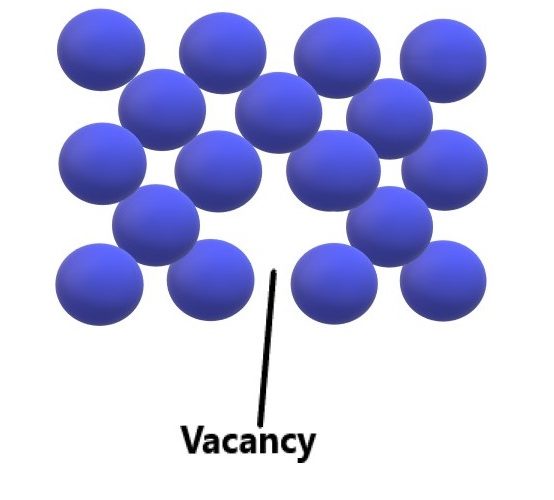
Vacancy defects: The most common defect is point defect. In any crystal when an atom leaves its position from its atomic site then the crystal is said to have vacancy point defect. Vacancy defects are formed during the solidification of crystal due to atomic vibrations and atomic rearrangements. These defects are also formed during any plastic deformation and ion bombarding. These defects are always in equilibrium and they require energy of 1 Electron volts for formation.
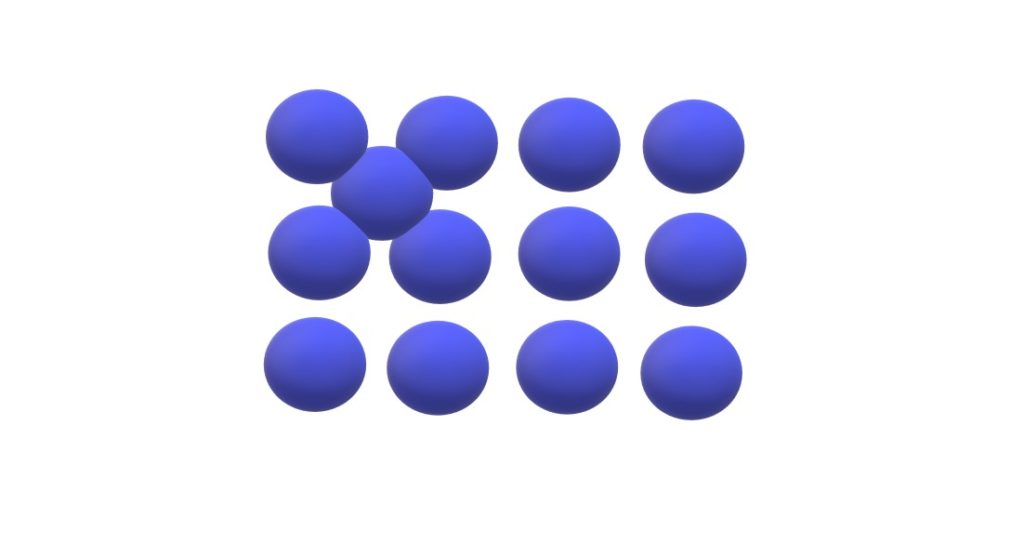
Self interstitial defect: In any crystal structure, arrangement of atoms is such that there are some gaps between the atoms which is called interstitial site and when any atom occupies interstitial site between then it is said to have self interstitial defect. Due to formation of these defects distortions in the nearby atoms is very large because the size of atom is comparatively large than the interstitial site.
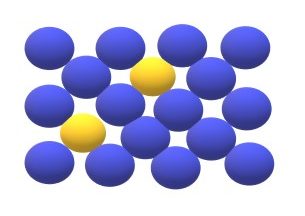
Substitution defect: In any solid solution when any impurity atom or solute atom takes the place of the parent atom than this type of defect is called substitution defect. In this type of defect structure of crystal remains same is diameter of the foreign atom is same as that of parent atom and if size of the foreign atom is bigger or smaller than that parent atom then the lattice may be distorted.
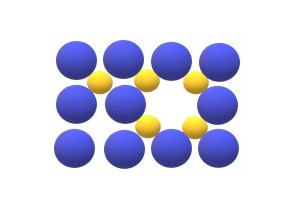
Schottky defects: Schottky explains the defects found in the ionic crystals, Defects in the ionic crystals is more complex because of necessity of maintaining electrical neutrality. According to Schottky defect when two oppositely charged ions are missing from an ionic crystal, a cation-anion vacancy is created which is called as
Schottky defects.
Frenkel defects: Same like Schottky, Frenkel gives explanation about defects in ionic crystals and according to Frenkel when a positive cation moves into an interstitial site in an ionic crystal , a cation vacancy is created in the normal ion site and this vacancy interstitialcy pair is called frenkel defect. Presence of these defects in ionic crystals increases the electrical neutrality.





Recent Comments