What is Boiling point | Boiling point of water |Explained
A substance’s boiling point is the temperature at which a liquid’s vapor pressure is equal to the pressure surrounding the material, and the liquid transforms into a vapour.
Surrounding environmental pressure affects the boiling point of substance and changes accordingly. Compared to the liquid in certain atmospheric pressure, a liquid in a partial vacuum has a lower boiling point.
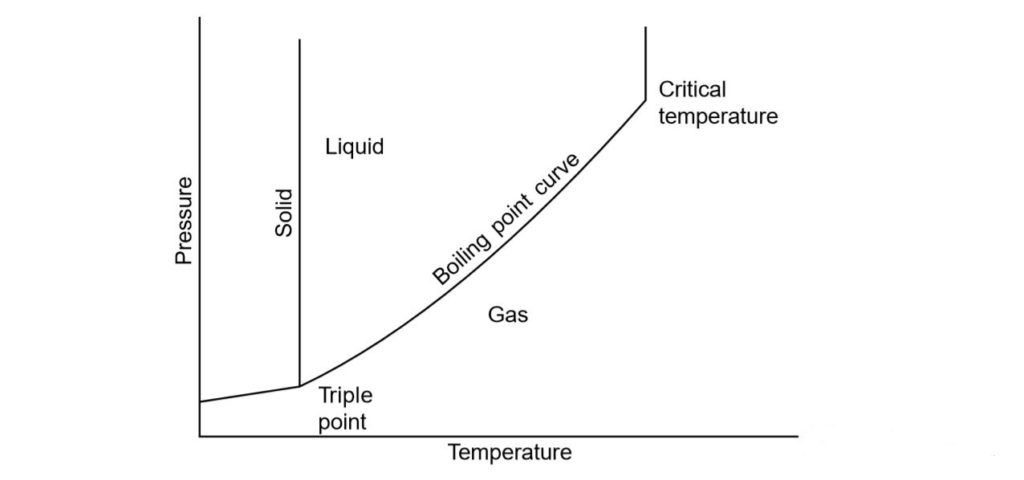
The temperature at which liquid is boiling is not constant, but differs with the pressure. Thus, though the boiling point of water is typically taken as 100 ° C, this is only true at a pressure of one normal atmosphere (1.013 bar) and the boiling point changes by varying the pressure
The boiling point is defined by the upper- critical temperature, above which it can’t exist as a liquid, and the lower end- triple point, at the freezing temperature. Under these two limits, if it is at pressure higher than its boiling pressure it will still remain in liquid condition and will be subcooled below the state of saturation, while if the temperature becomes higher than saturation, it will be a gas and superheated.
When both liquid and vapour are in the same enclosure and there is no other volatile material, the state must be on the line of saturation.The solid can change directly to a gas (sublimation) at a pressure below the triple point pressure and the gas can change directly to a solid, as in carbon dioxide snow produced from the released gas.




Recent Comments