Fick’s law of diffusion in solids
Adolf Fick gave law of diffusion in fluids and solids. Initially Fick’s law of diffusion were only for fluids but later on it was seen that Fick’s law were applicable for solids also.
According to Fick’s law, diffusion is a time dependent process i.e. in a microscopic sense the quantity of an element that is transported within another is a function of time so he derived two laws called Fick’s first law or Steady state diffusion and Fick’s second law or Non-steady state diffusion. Now let us understand one by one.
Fick’s law of diffusion (First law):
Fick’s first law is also termed as steady state low and according to this law flux flows from high concentration region to low pressure region with the pressure equivalent to the concentration gradient or in other words flux is directly proportional to the concentration gradient.
This type of diffusion occurs when a non reacting gas diffuses through a metal foil for example This type of diffusion takes place when hydrogen gas diffuses through palladium because hydrogen gas is at high pressure on one side and at low pressure on other side. Such type of diffusion is also called steady state diffusion of Fick’s law.
If in the diffusion system no chemical reaction occurs between the solute and solvent atoms because there is a concentration difference between the planes so there will be net flow of atoms from the higher concentration to lower concentration which is expressed as flux (J).
The Flux is defined as the number of atoms passing through a plane of unit area perpendicular to direction of diffusion per unit.The flux in this type of system can be expressed as:
J= -D. dc/dx
Where D is the diffusivity ( cm2/s ) and dc/dx is the concentration gradient (atoms/ cm3 .cm). A negative sign is used because the diffusion is from a higher to lower concentration, Making the dc/dx term negative. This equation us called Fick’s first law of diffusion and it states that for steady state diffusion conditions, the net flow of atoms by atomic diffusion is equal to the diffusivity D times the diffusion gradient dc/dx.
Fick’s law of diffusion (Second law):
Fick’s law of Steady state diffusion in which concentration do not change with time is not commonly encountered with engineering materials. In most cases, non-steady-state diffusion in which concentration of solute atoms at any point in the materials changes with time. For non-steady-state diffusion in which the diffusivity is independent of time, Fick’s second law of diffusion applies, which can be represented as
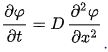
Where,
D, is the diffusion coefficient. x, is the position. t, is the time
This law states that the rate of compositional changes is equal to diffusivity times the concentration gradient and is also called as non- steady state diffusion of Fick’s law.





Recent Comments