Thermodynamic system and its types | Explained
A system is defined as region of space or quantity of matter enclosed by boundary. This boundary may be fixed or movable as in case of internal combustion engines piston moves boundary back and forth. System can be completely closed or can have path for flow of matter in or out of system. Space enclosing the system is called the boundary and Area other than system or outside the boundary is called surrounding.
Types of thermodynamic system
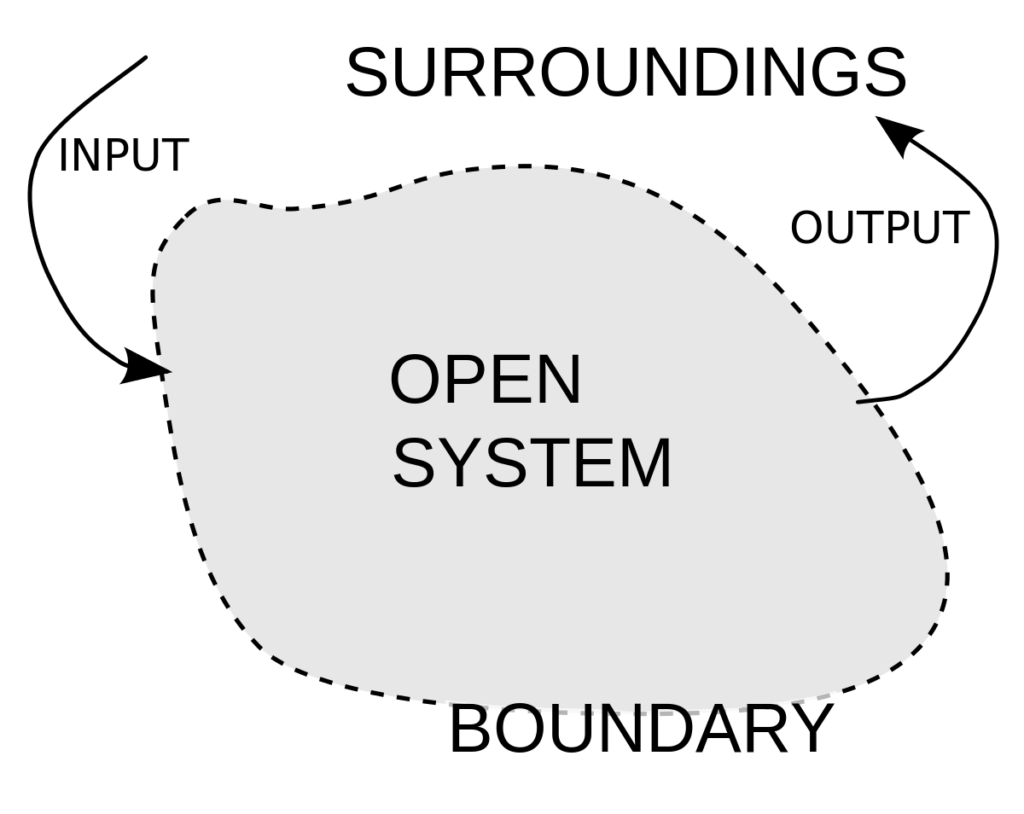
1. Open system
Open system is the type of thermodynamic system in which boundary of system has path to flow matter in and out from surrounding. Most of engineering systems are of open type. One of example of open thermodynamic system is Internal combustion engine
2. Closed system
In Closed system boundary of system is enclosed to flow of matter from and to surrounding. There is no transaction of matter between system and surrounding. Example of closed system is boiler tank having no inlet and outlet holes.

3. Isolated system
In isolated system boundary is such that there is no exchange of matter and energy between system and surrounding. Example of isolated system is Complete insulated tank restricted to flow of matter and energy.
4. Adiabatic system
An Adiabatic system is one which is thermally insulated from surrounding however it can exchange work with its surrounding. If it does not exchange the work with surrounding it becomes an isolated system.
5. Homogeneous system
Homogeneous system is the one in which matter is present in one phase only i.e. Matter is homogeneous throughout is composition and physical structure.
6. Heterogeneous system
A system in which Matter is present in more than one phase then it is called a heterogeneous system. Example of a heterogeneous system is water and steam.






Recent Comments