Gibbs Phase Rule Explained With Example
J W Gibbs gave an equation from thermodynamic calculations and consideration that derives the number of phases that can co-exist in equilibrium in a chosen system to be computed. This equation is called Gibbs phase rule and can be represented as follows
P + F =C + 2
Where C is the number of components in a system , P is the number of phases which coexist in a chosen system and F is the degree of freedom.
A phase is a region of system with the same structure and uniform composition and differs from other regions of the system either in structure and composition.
The component is a chemical constituent of a system , which may be used to specify its composition.
For example, Let us apply the Gibbs phase rule and calculate the degree of freedom of water. This will be calculated at the triple point of pressure – temperature phase diagram of water as shown in below diagram. At the triple point, three phases coexist in equilibrium, thus P=3 and since there is one component in system so there fore C = 1, the number of degree of freedom can be calculated as under
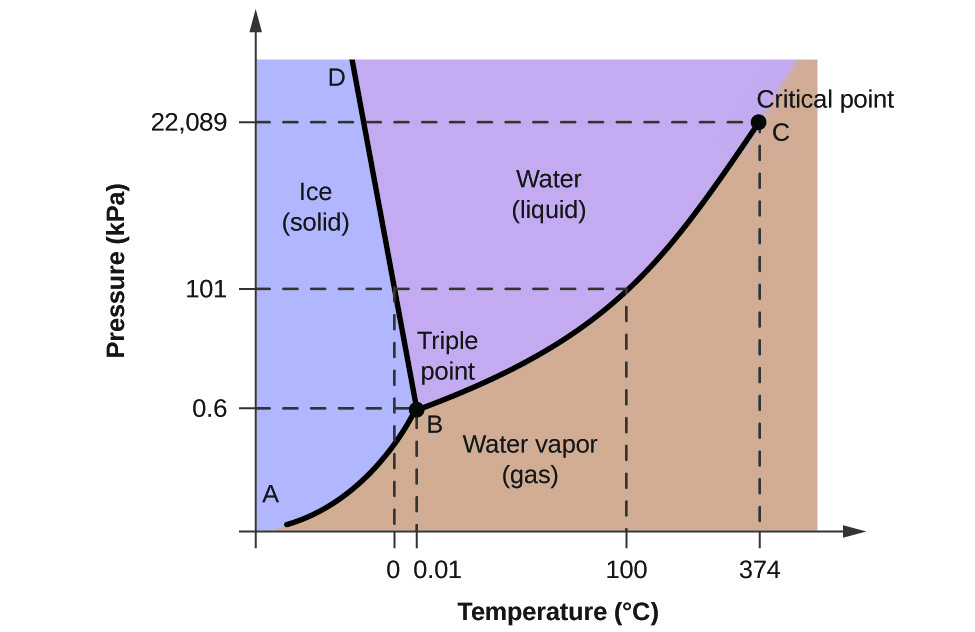
P + F = C + 2
3 + F = 1 + 2
F = 1 + 2 – 3
F = 0 (Zero degree of freedom)
Since none of the variables can be changed, The triple point is called a Invariant point.



